Electrolyzer
What is an electrolyser and why is it key to green hydrogen supply?
The electrolyser is an apparatus that produces hydrogen through a chemical process (electrolysis) capable of separating the hydrogen and oxygen molecules of which water is composed using electricity. Hydrogen produced in this sustainable way, i.e. without emitting carbon dioxide into the atmosphere, can be the basis for a decarbonised economy.
Electrolysis may sound at first like a high school laboratory experiment with beakers, a few wires and a couple of batteries, and we would not be wrong. But the impact of this process, which allows molecules to be broken down using electricity, in this case water molecules, is key to obtaining green hydrogen.
Hydrogen generation
Hydrogen is the most abundant element in the universe and can therefore become the perfect fuel. But this is not the only reason: when hydrogen is burned, carbon dioxide is not produced; instead, water vapour is produced. In this way, its use would drastically reduce the emissions responsible for the greenhouse effect and global warming.
The difficulty lies in the fact that in order to obtain hydrogen, electrical energy is needed, and if this energy comes from fossil fuels, emissions would be generated. In contrast, the production of what is known as green hydrogen is based on the use of renewable energies to power the electrolysis process, by means of which hydrogen is obtained from water. The machine in charge of this process is called an electrolyser.
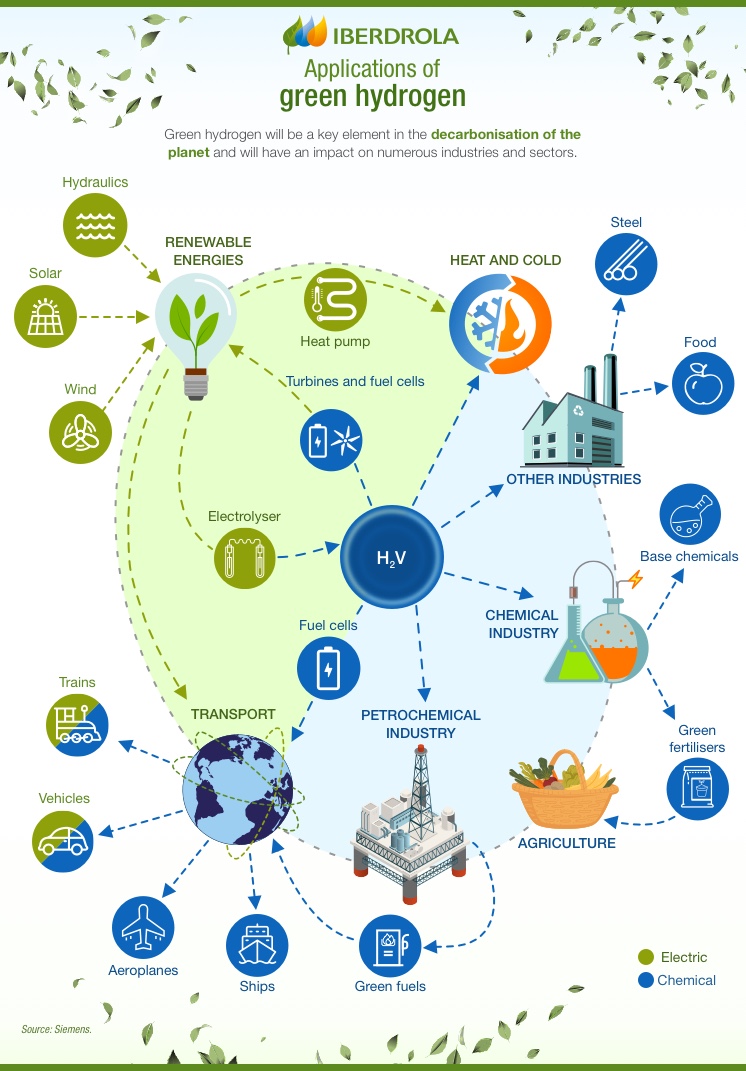
 SEE INFOGRAPHIC: Applications of green hydrogen [PDF] External link, opens in new window.
SEE INFOGRAPHIC: Applications of green hydrogen [PDF] External link, opens in new window.
What is an electrolyzer and how it works: elecrolysis
An electrolyser is a device capable of splitting water molecules into their constituent oxygen and hydrogen atoms. The bonds between the two elements are very stable and electrical energy is needed for this splitting to take place in a process called electrolysis [PDF] External link, opens in new window.. Efficient electrolysers will be key to the penetration of hydrogen in industries and the adoption of hydrogen fuel cells.
One of the world's largest electrolysers is located in Fukushima, Japan, at the site of the well-known nuclear disaster, symbolising a paradigm shift in energy production as it is powered by solar panels. Most recently, in January 2021, the Japanese electrolyser was far surpassed by the one in Bécancour, Canada, which consists of a polymer membrane device with an output of 8.2 tonnes per day.
How an electrolyser works
Electrolysis was first discovered in 1800. After the invention of the electric battery by Alessandro Volta in the same year, other chemists tried connecting their poles in a container of water. They discovered that the current flowed through the water and that hydrogen and oxygen were separated at the electrodes.
An electrolyser consists of a conductive electrode stack separated by a membrane to which a high voltage and current is applied. This causes an electric current in the water which causes it to break down into its components: hydrogen and oxygen. The complete system also includes pumps, power electronics, gas separator and other auxiliary components such as storage tanks.
The oxygen generated in parallel is released into the atmosphere or can be stored for later use as a medical or industrial gas in some cases. The hydrogen is stored as a compressed gas or liquefied for use in industry or in hydrogen fuel cells, which can power transport vehicles such as trains, ships and even aircraft.

Green hydrogen
An alternative that reduces emissions and cares for our planet.

Hydrogen stations
What are hydrogen stations and how do they work?

Difference between green and blue hydrogen
Discover the importance of the colours of hydrogen.

Green ammonia
The sustainable revolution in the chemical industry.
Types of electrolyzers
At present, there are different types of electrolysers depending on their size and function. The most commonly used are:
Alkaline electrolyser
They use a liquid electrolyte solution, such as potassium hydroxide or sodium hydroxide, and water. Hydrogen is produced in a cell consisting of an anode, a cathode and a membrane. The cells are usually assembled in series to produce more hydrogen and oxygen at the same time. When current is applied to the electrolysis cell stack, hydroxide ions move through the electrolyte from the cathode to the anode of each cell, generating bubbles of hydrogen gas on the cathode side of the electrolyser and oxygen gas at the anode. They have been in use for more than 100 years and do not require noble metals as a catalyst; however, they are bulky equipment that obtains medium purity hydrogen and are not very flexible in operation.
Proton exchange membrane (PEM) electrolyser
PEM electrolysers use a proton exchange membrane and a solid polymer electrolyte. When current is applied to the battery, water splits into hydrogen and oxygen and the hydrogen protons pass through the membrane to form hydrogen gas on the cathode side. They are the most popular because they produce high-purity hydrogen and are easy to cool. They are best suited to match the variability of renewable energies, are compact and produce high-purity hydrogen. On the other hand, they are somewhat more expensive because they use precious metals as catalysts.
Solid oxide electrolysis cell (SOEC)
SOECs operate at a higher temperature (between 500 and 850 ºC) and have the potential to be much more efficient than PEMs and alkaline electrolysers. The process is called high-temperature electrolysis (HTE) or steam electrolysis and uses a solid ceramic material as the electrolyte. Electrons from the external circuit combine with water at the cathode to form hydrogen gas and negatively charged ions. Oxygen then passes through the sliding ceramic membrane and reacts at the anode to form oxygen gas and generate electrons for the external circuit. Technologically they are less developed than the above.
There are other types of electrolysers that are not yet as efficient or cost-effective as the above, but have a lot of potential for development. One example is photoelectrolysis, which uses only sunlight to separate water molecules without the need for electricity. However, this device requires semiconductors that have not yet been sufficiently developed.




