Lithium-ion batteries
Lithium-ion batteries, essential for energy storage
The future of decarbonisation depends on effective energy storage, among other factors, whether on a small scale in, for example, an electric car, or on a large scale in the distribution network. This is where lithium-ion batteries, currently the most competitive, come into play. Here, we take a look at their components, how they work, their advantages and their role in a sustainable future.

Your wireless headphones, your mobile phone, your smart watch, your solar panel installation or your electric car would not have been possible just a couple of decades ago. This revolution has come about thanks to, among other things, lithium-ion batteries. These batteries are capable of storing more energy in less space than others and will therefore be key to the future of energy storage in the face of the challenges of climate change, which include decarbonisation and renewable energies.
The cost of lithium-ion batteries has fallen by 85 % since 2010 and is expected to fall further over the next decade. According to Rory McCarthy, an energy storage analyst with Wood Mackenzie, "lithium-ion has a significant advantage over other alternative storage technologies, and that is economies of scale". In other words, its progressive adoption is driving down costs. Even despite the general increase in 2022, prices fell rapidly again the following year, reaching an all-time low in 2023. This trend is expected to continue.
What is a lithium-ion battery
A lithium-ion or Li-Ion battery is a type of rechargeable battery that uses lithium compounds as one of the electrodes. In 1985, Akira Yoshino developed the first prototype based on earlier research by John Goodenough and other experts during the 1970s. Subsequently, a Sony team developed the first commercial lithium-ion battery in 1991. Further advances were made over the years, especially in the use of nickel-manganese-cobalt oxide (NMC) cathodes, which improved charge density, performance and safety.
Charging lithium-ion batteries, functioning and characteristics
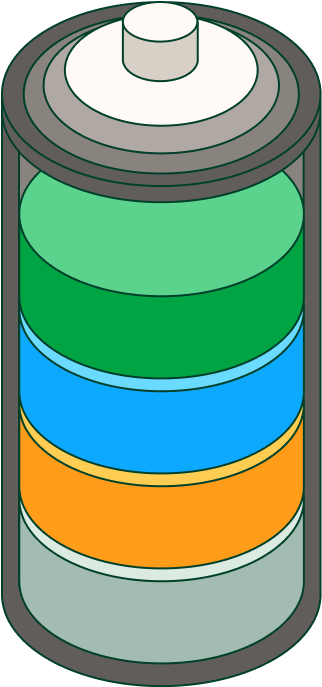
Lithium-ion batteries are made up of the following parts: a negative electrode or anode from which the electrons are released and a positive electrode or cathode that receives them. When the battery is connected, lithium ions move from the anode to the cathode through an electrolyte, resulting in the potential difference that produces the current. When the battery is charged, the lithium ions return to the anode.
In turn, batteries are made up of one or more cells and, depending on their end use, there are different types: cylindrical cells, which are used in most electric vehicles, consist of sheets of different components that are rolled into a cylinder, while flat cells, such as those found in mobile phones and laptops, use lithium-ion polymer in the form of stacked sheets.
In addition, lithium-ion batteries incorporate other elements that improve their performance and safety: a temperature sensor, a voltage regulator circuit and a state-of-charge monitor. These components monitor the charge and current flow, record the last capacity reached at full charge and monitor temperature, which can negatively affect battery life.
Tips for extending the life of lithium-ion batteries
Minimise exposure to high temperatures in use or storage.
Minimise use at low temperatures, especially during charging.
Minimise charge time to 100%.
Minimise charge time to 0%.
Avoid the use of fast charge unless necessary.
Avoid discharging the device faster than necessary.
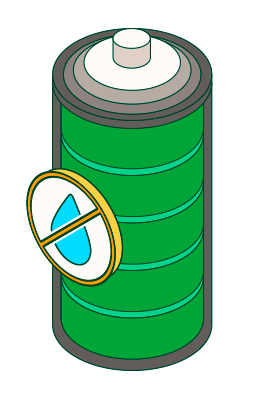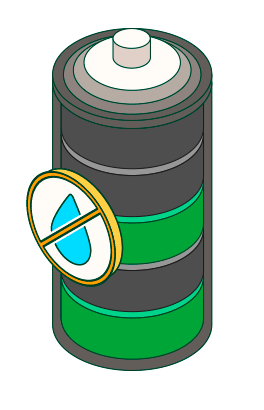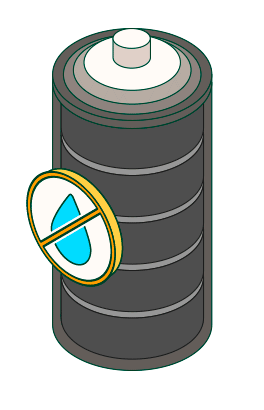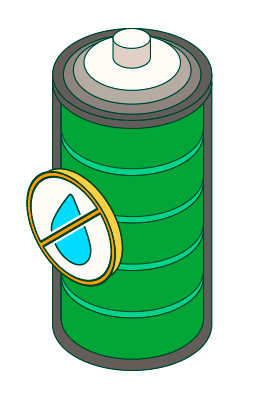
Avoid use or storage in humid environments.
Follow the manufacturer’s calibration instructions.
Source: University of Michigan
 SEE INFOGRAPHIC: Advice for extending the useful life of lithium-ion batteries [PDF] External link, opens in new window.
SEE INFOGRAPHIC: Advice for extending the useful life of lithium-ion batteries [PDF] External link, opens in new window.
Advantages and disadvantages of lithium-ion batteries
Compared to traditional nickel hydride or nickel-cadmium rechargeable battery technology, lithium-ion batteries have several advantages: primarily, they charge in less time and take longer to discharge, but they also have a higher energy density, have no memory effect and lose virtually no charge when not in use, etc.
However, like any technology, they have certain disadvantages mainly related to protection (they must incorporate systems to prevent overcharging and overheating) and cost (lithium batteries are still slightly more expensive than nickel-cadmium batteries at the outset, although they are more cost effective by a small margin).
Applications of lithium-ion-batteries
The advantages of lithium-ion batteries and their decreasing cost have led to a proliferation of their use in many areas:
 Emergency power systems
Emergency power systems
In critical installations, such as server farms, the batteries of a UPS (Uninterruptible Power Supply) protect you from the loss or instability of the electricity supply.
 Solar energy storage
Solar energy storage
Solar energy storage is intermittent and these batteries are best suited to solar panels because of the way they charge and their speed, especially for self-consumption.
 Consumer electronics and mobile devices
Consumer electronics and mobile devices
Mobile devices have become the main application for these batteries, allowing for ever-increasing miniaturisation.
 Disability assistance
Disability assistance
These types of batteries are present in electric wheelchairs, stair lifts or motorised prostheses, making life easier for people with mobility restrictions.
Lithium-ion batteries for electric vehicles
The development and increasing adoption of electric and hybrid vehicles is largely due to the efficiency and lower cost of lithium-ion batteries. In addition to having a high energy density compared to their size, their mass production has brought the price of electric vehicles closer to petrol-powered vehicles. In terms of operating costs, the price of electricity to run an electric vehicle is lower than the cost of fuel for internal combustion engines. Also provide autonomy for longer and longer journeys.
The storage of the future
The shortage of cobalt, a necessary material to manufacture them, and the difficulties in recycling it are driving research into alternative battery technologies. Here are some of the most promising:
- Hydrogen cells: these batteries produce electricity from gaseous hydrogen, but the drawback is in being ab to make green hydrogen without resorting to fossil fuels, not the technology itself.
- Solid-state batteries: use solid instead of liquid or gel electrolytes. They have a higher energy density, reduce the risk of explosion and fire and take up less space because they don’t require as many safety-related components.
- Graphene supercapacitors: Capacitors can be charged and discharged more efficiently than a battery and the use of graphene could achieve energy densities similar to current batteries.





